Macrophage Biology and Mechanisms of Cellular Uptake
Macrophage Biology: Macrophages are fascinating, highly plastic cells, which perform critical functions in homeostasis and disease. They are very motile, and can seek out, bind, engulf, and destroy pathogens like bacteria and viruses. But they also serve important roles in normal housekeeping, removing dead cells and debris. During neuronal development, microglia (the macrophages of the brain) help prune synapses, while defective macrophage function is implicated in the pathogenesis of neurodegenerative diseases where failure to remove protein aggregates can be detrimental.
At the same time, macrophages can adopt dramatically different cell states depending on their microenvironment, shifting from phagocytic clearance to wound healing functions, with many sub-states in between. The metabolic demands on a macrophage after its engulfment of a target cell also induce significant perturbations to its state. We are very interested to understand how all of these states are managed, and how macrophage function is controlled via signaling interactions with other cells in the environment of tumors, pathogens, and neurotoxic aggregates.
Phagocytosis of diverse particles: How do macrophages recognize and engulf such diverse particles? To address this, we developed a system where magnetized particles are ‘eaten’ by macrophages, enabling systematic surveys of the genetic requirements for each (a wonderful collaboration with the Barres lab). This has revealed a wealth of new insights, and pointed to a number of candidate therapeutic targets for cancer and neurodegeneration.
Tumor-macrophage interactions: Macrophages are capable of recognizing and killing tumor cells, particularly with the help of tumor-targeting antibody therapies, where macrophages often contribute significantly to reducing tumor burden and can also cross-present antigens to activate other arms of the immune response. Remarkably, sometimes up to 50% or more of the tumor mass is comprised of macrophages. However, these are typically ‘M2-polarized’ and are not killing tumor cells, but instead secreting cytokines that can support tumor growth. We would like to understand this altered state, and how to reverse it to improve therapies.
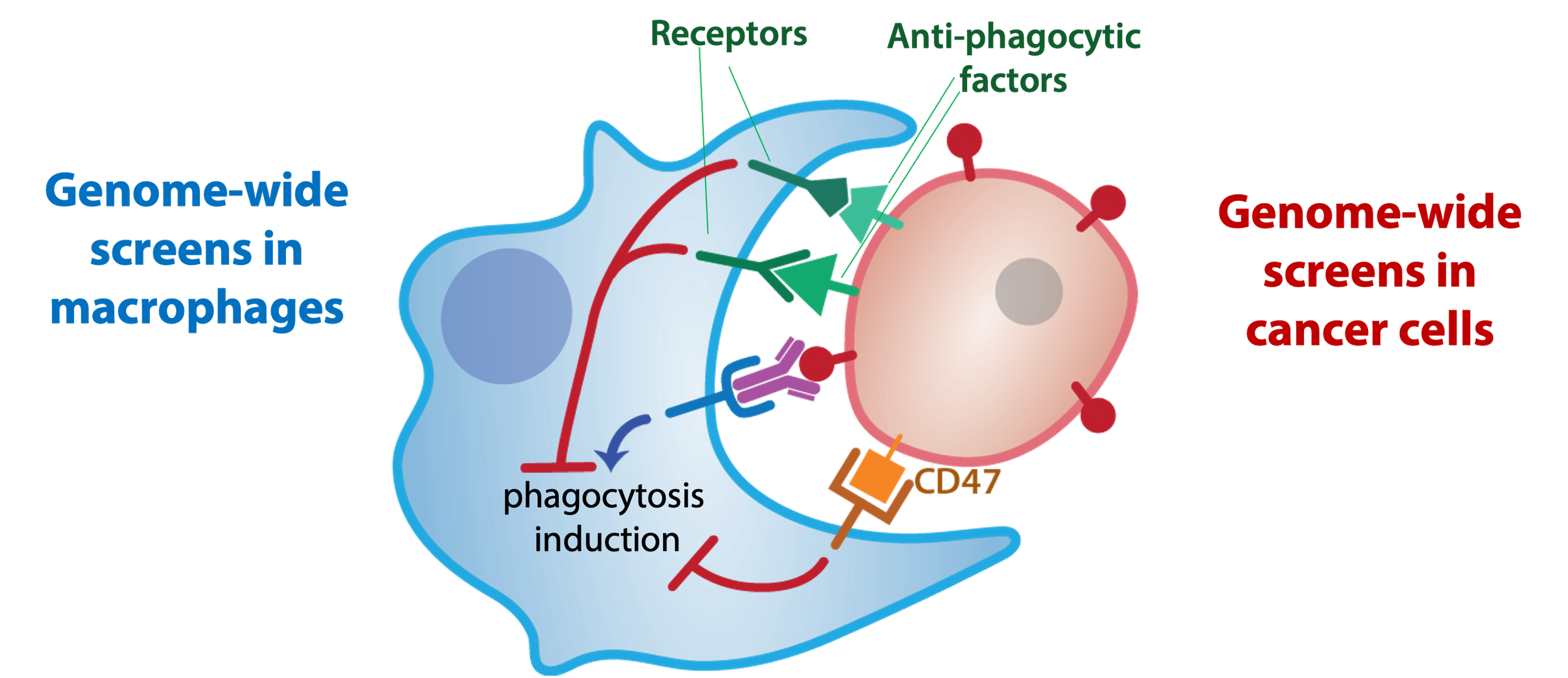
Tumors also upregulate so called ‘don’t-eat-me’ signals, e.g. CD47 (first characterized by Irv Weissman and colleagues), that prevent macrophages from killing them. We recently developed a platform for inter-cellular CRISPR screens to identify regulators of tumor-macrophage interactions, which revealed a host of new therapeutic targets to enhancer tumor killing by macrophages.
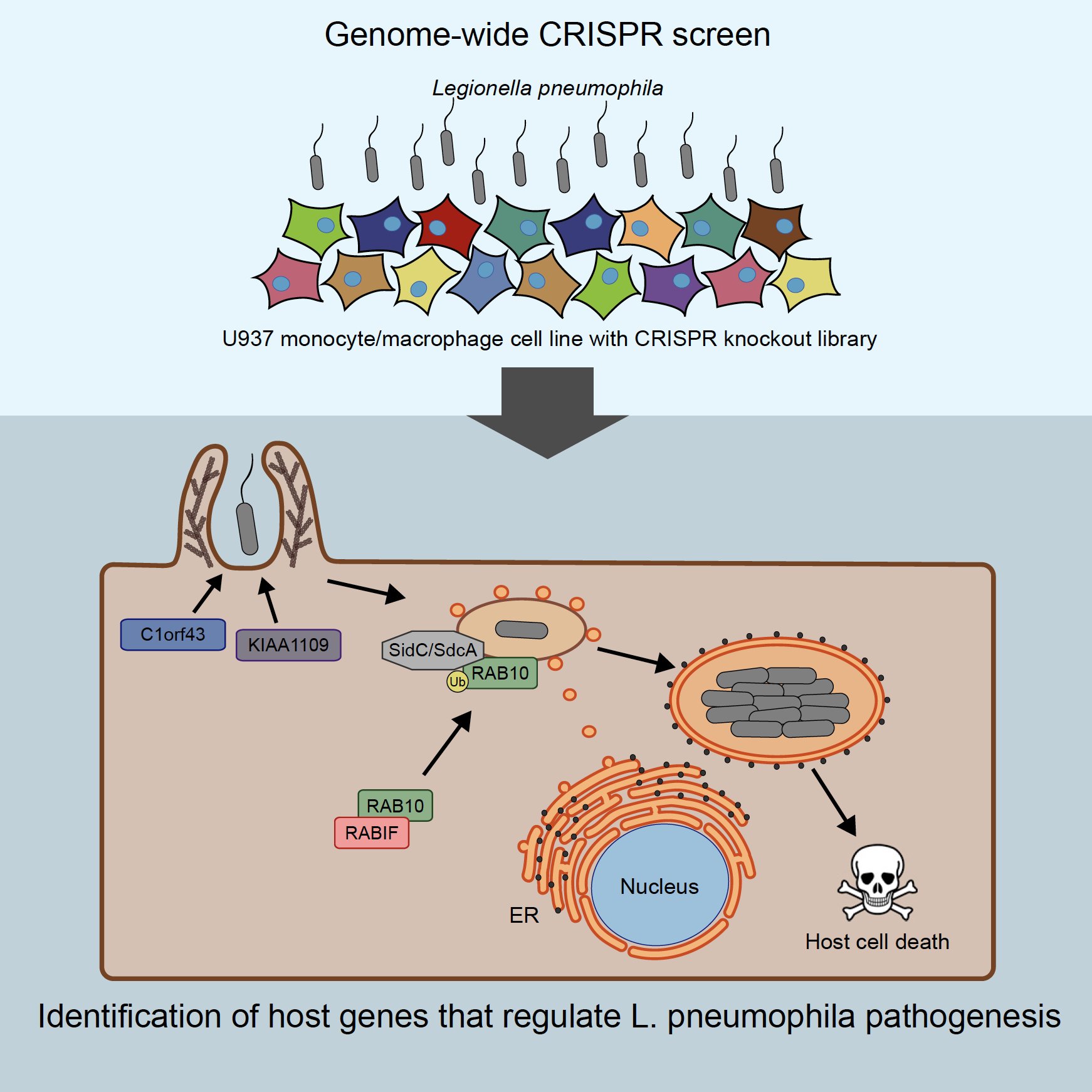
Macrophage interactions with bacteria and viruses: Bacteria and viruses have evolved remarkably complex ways to enter the cell, disrupt homeostasis, and cause cell death. We are interested in studying these agents both as probes to understand normal cellular trafficking and signaling events, and to find key targets for therapy. For example, working with the Mukherjee lab, we identified factors controlling the phagocytosis and proliferation of Legionella bacteria. In other studies, we’ve examined how very different particles such as neurotoxic protein aggregates are taken up by cells to induce neurodegeneration.